Food and Drug Administration on Thursday recommended a half-dose booster shot of the Moderna vaccine be given to certain recipients six months. We are pleased to initiate the submission process for our booster.
Moderna Has Asked The Fda To Authorize A Booster Of Its Covid 19 Vaccine Wxxi News
A key FDA advisory committee unanimously recommended Thursday giving booster shots of Modernas Covid-19 vaccine to people ages 65 and older and other vulnerable Americans.
Moderna booster shot. Heres the latest news for prior vaccine recipients The FDA may soon approve a Moderna booster after a unanimous vote by the Vaccines and Related Biological Products. FDA authorizes Moderna JJ vaccine boosters 0229. FDA authorizes booster shots of Moderna Johnson Johnson Covid-19 vaccines.
In the end the FDA. Per YahooNews FDA scientists released new documents that show the Moderna vaccines booster shot created antibodiesBut the difference in antibody levels before and after the booster shot wasnt big enough to warrant a booster shot. As the pandemic continues to impact the country science has shown that vaccination continues to be the safest and most effective way.
But Moderna only sought permission for a half-dose as a booster shot submitting data in support of that. Decided to authorize the half-dose Moderna booster. Early signs may favor the booster dose for Modernas COVID-19 vaccine.
People who received either the Moderna or Pfizer vaccine and are eligible for a booster shot may receive a dose of Moderna Pfizer or Johnson Johnson at least six months after being fully vaccinated according to the FDA. A panel of independent experts convened by the Food and Drug Administration voted unanimously Thursday. With those data in hand CDC will keep the public informed with a timely plan for Moderna and JJJanssen booster shots.
A federal committee is expected to vote Thursday on whether to allow booster shots for the Moderna and Johnson Johnson COVID-19 vaccines following the Food and Drug Administrations. Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. Will the Moderna booster be the same as the two Moderna COVID-19 shots.
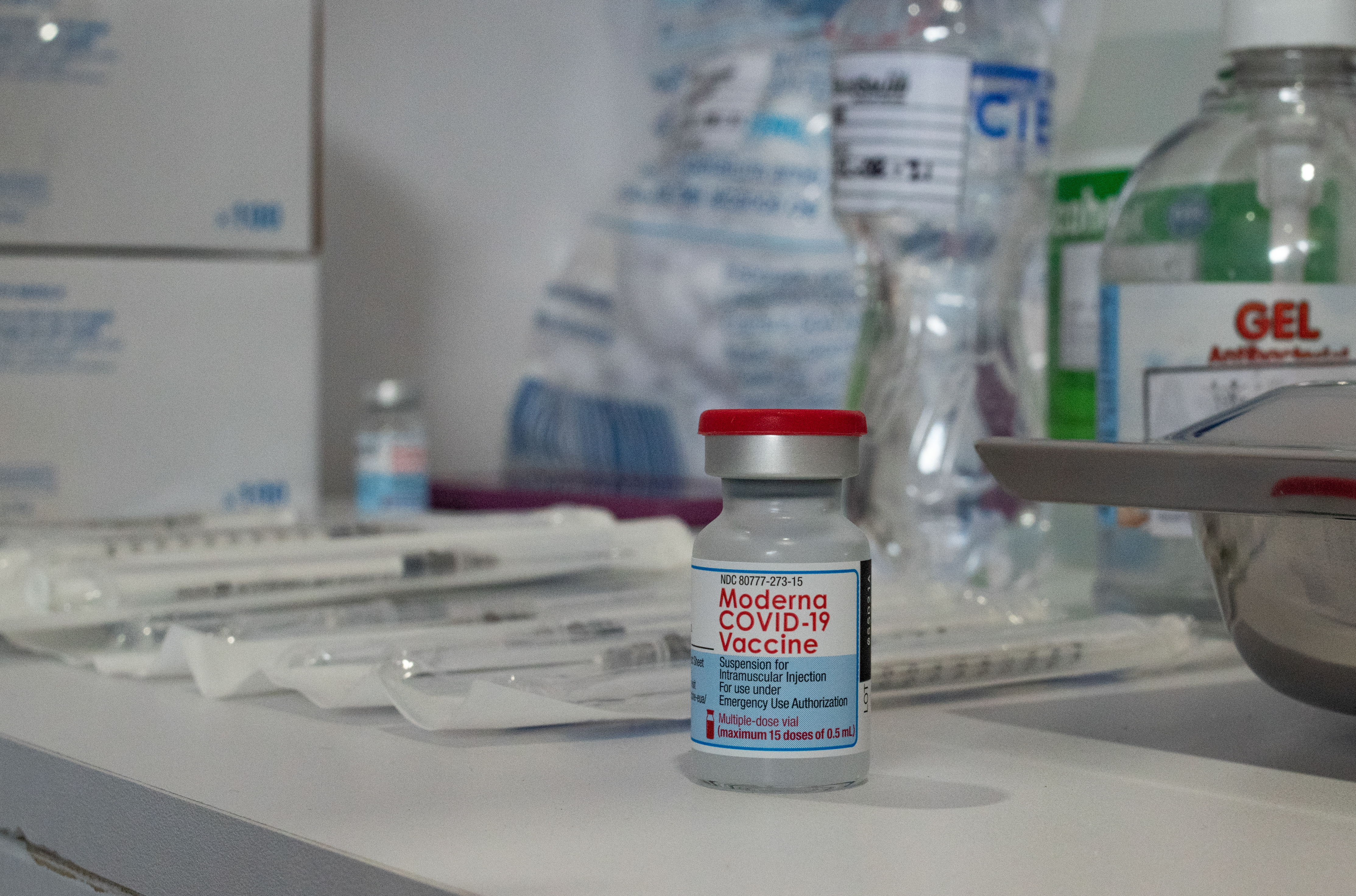
A vial of the Moderna COVID-19 vaccine is seen at a local clinic as the spread of the coronavirus disease. The FDA panel wrestled with whether Moderna presented enough evidence backing its low-dose booster. Moderna COVID vaccine booster shot receives recommendation but its not a done deal.
Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. M any Americans who have been vaccinated against Covid-19 will soon be able to choose which vaccine they would like as. To support the authorization for emergency use of a single booster dose of the Moderna COVID-19 Vaccine the FDA analyzed immune response.
Moderna COVID vaccine booster shot gets an early thumbs-up but its not a done deal. Moderna booster shot update. The FDA on Wednesday granted emergency use authorization to vaccine booster shots from Moderna and Johnson Johnson clearing a key regulatory.
FDA scientists on Tuesday declined to take a stance on whether to back booster shots of Modernas Covid-19 vaccine. Early signs may favor a booster dose for Modernas COVID-19 vaccine. But Moderna says a single 50-microgram shot should be enough for a booster.
Coronavirus Updates Moderna submitted data from 344 volunteers who got a third shot of the vaccine six months after their. Scientists with the Food and Drug Administration said Tuesday that Moderna did not meet all the criteria necessary for the FDA to support a booster vaccine. As with Pfizers booster the third Moderna shot will be the same vaccine as the first two doses except itll.
EU regulator verdict on Moderna COVID-19 booster shot next week. Mirroring a similar recommendation issued last month for the Pfizer COVID-19 vaccine an expert advisory panel to the US. More data on the effectiveness and safety of Moderna and JJJanssen booster shots are expected soon.
They said data shows two doses are still enough to protect against severe. The endorsement is a. In a preprint study Moderna reported that its COVID-19 vaccine protection drops 36 after 12 months which argues for a booster shot for all.
Moderna just got a step closer to rolling out a COVID-19 booster shot to the general public. Moderna Asks FDA To Authorize A Booster Shot Of Its COVID-19 Vaccine. COVID-19 vaccines are working well to prevent severe illness.
Our submission is supported by data generated with the 50 µg dose of our COVID-19 vaccine which shows robust antibody responses against the Delta. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA. As the delta variant.
If we need a booster shot does that mean that the vaccines arent working. The effectiveness of the two-dose vaccine against infection wanes over time according to data the CDC posted. FDA Panel Supports Moderna Booster Shot for Older Adults People at High Risk.
Authorization of Moderna COVID-19 Vaccine Booster Dose. Moderna recipients can get a booster at half the dosage of the original two shots.

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times
Moderna Says Higher Infection Risk After One Year Supports Covid Boosters Barron S
Moderna Looks To Test Covid 19 Booster Shots A Year After Initial Vaccination

Covid Booster Shots Everything You Need To Know The Brink Boston University

Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants

European Agency Clears Moderna Vaccine For Children 12 17 Wvns

Seeking Approval For A 3rd Dose Pfizer And Biontech Take Their Covid 19 Booster Data To The Fda Fiercepharma

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News
Covid Vaccine Ema Backs Pfizer Or Moderna Booster Shots For People With Weak Immunity Euronews

Moderna Is Developing Vaccine Booster Shot To Fight South Africa Covid Strain Youtube

Frequently Asked Questions And Answers About Moderna Covid 19 Vaccine Bangkok Hospital

People Are Getting Moderna Boosters Anyway Medpage Today
Moderna Covid Vaccine Booster Shot The Latest On Approval Process Cnet
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/gray/NZPGPXPQUJDSVC7FJIONPNSKWU.jpg)
Can People Who Received Moderna Or Johnson Johnson Get A Pfizer Booster Shot Health Officials Explain

Moderna Asks Health Canada To Authorize Booster Shot Of Its Covid 19 Vaccine Times Colonist

Moderna To Supply 20 Mln Doses Of Covid 19 Vaccine To Peru Reuters
YOU MAY LIKE :




